EFPIA / EFSPI Estimand Implementation Working Group (EIWG)

Charter & Purpose
“EIWG brings together statisticians and clinicians to support the estimand journey”
- To provide a cross-industry forum to:
- share Industry and Academic experiences of implementing the new estimand framework introduced in ICH E9(R1)
- discuss issues emerging through implementation
- be champions and engage in scientific discussion about the value and benefits of the framework
- With the aim to:
- give feedback and recommendations for best practices
- promote broad understanding and awareness of the framework within and outside of statistics
- consolidate issues and topics for discussion with the ICH E9 Implementation Working Group
- provide training through case studies, e.g., through the EIWG Estimand Training Academy
EIWG Estimand Training Academy
- Targeted to anyone working in clinical trials: Clinician, Regulator, Investigator, Academic, Ethics Committee, HTA Agencies, Statistician.
- All four trainings are freely available as ‘Video-on-Demand’
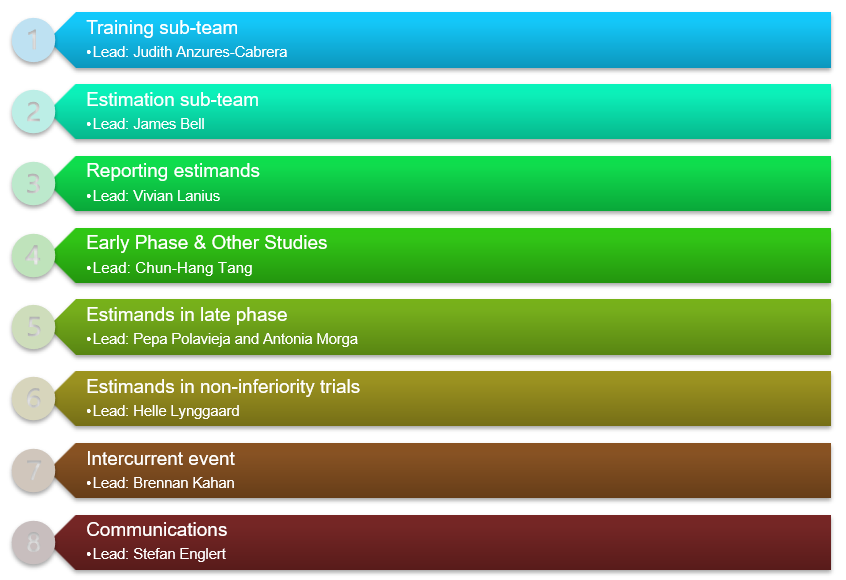
2024 EIWG Sub-teams
2024 Ongoing/planned Activities
- Training sub-Team
- Planning on creating estimand webinars on neuroscience, early development and HTA
- Estimation sub-team
- Publication on treatment policy estimation
- Reporting Subteam
- Publication on the reporting of estimands and intercurrent events in clinical study reports
- Successful collaboration with NIC on the reporting of estimands on “Clinicaltrials.gov”
- Estimands in Early Phase [I/II] & Clinical Pharmacology Studies sub-team
- Develop a case study for implementing estimands for a dose-ranging (Ph2b) study
- Develop a case study with focus on clinical pharmacology studies in pregnant and breast-feeding women
- Input into guidance around implementing estimands for patient-reported outcomes in early phase trials
- Explore estimands for concentration modelling for PD effect (e.g., concentration-QTc).
- HTA and RWE sub-team
- Non-inferiority sub-team
- PSI session on estimands in non-inferiority trials
- First paper published
- Paper initiated on case studies illustrating challenges discussed in first paper
- Intercurrent Event sub-team
- Continue to identify areas of uncertainty around how the different intercurrent event strategies may be interpreted
- Identify areas of uncertainty around whether certain events meet the definition of an intercurrent event
- Identify and begin to implement appropriate strategies to reduce this uncertainty, e.g. through training materials, a possible publication, or feeding back to the ICH E9(R1) creators
- Communications sub-team
- Establish an EIWG teams site for efficient communication and collaborative working
- Homepage with working group updates and estimand resources, hosted and managed by the EIWG
- PSI session on estimand thinking and statistical leadership
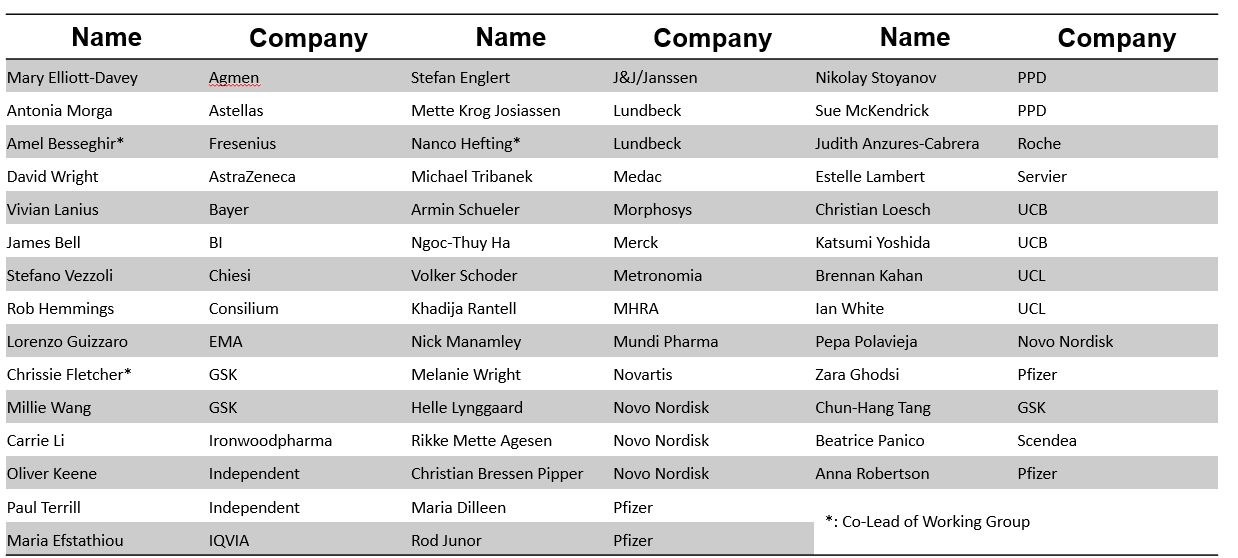
EIWG Members
The EIWG consists of 43 members representing 26 companies and institutions
The sub-team is planning to prepare a manuscripton these topics.
Publications
- D. Wright, H. Lynggaard , S. Englert, V. Lanius, O. Keene. Why Estimands are Needed to Define Treatment Effects in Clinical Trials. BMC Medicine 21:276 (2023)
- A. Morga, NR. Latimer, M. Scott, N. Hawkins, M. Schlichting, J. Wang. Is Intention to Treat Still the Gold Standard or Should Health Technology Assessment Agencies Embrace a Broader Estimands Framework?: Insights and Perspectives From NICE and IQWiG on the ICH E9(R1) Addendum. Value Health. 2022 Sep 20:S1098-3015(22)02148-9. https://doi.org/10.1016/j.jval.2022.08.008
- H. Lynggaard, J. Bell, C. Lösch, A. Besseghir, K. Rantell, V. Schoder, V. Lanius. Principles and Recommendations for Incorporating Estimands into Clinical Study Protocol Templates. Trials (2022) https://doi.org/10.1186/s13063-022-06515-2
- O. N. Keene, D. Wright, A. Phillips, M. Wright. Why ITT analysis is not always the answer for estimating treatment effects in clinical trials (2021). Contemporary Clinical Trials. https://doi.org/10.1016/j.cct.2021.106494
- C. Fletcher, N. Hefting, M. Wright, J. Bell, J. Anzures-Cabrera, D Wright, H. Lynggaard, A. Schueler. Marking 2-years of new thinking in clinical trials - the estimand journey. Ther Innov Regul Sci 56, 637–650 (2022). https://doi.org/10.1007/s43441-022-00402-3
Partnerships with related groups
- European special interest group “Estimands in oncology”, sponsored by PSI and EFSPI and ASA scientific working group of the ASA biopharmaceutical section
- Mission: The cross-industry working group (www.oncoestimand.org) was initiated to foster a common understanding and consistent implementation of the estimand framework in oncology clinical trials.
- Liaison: Stefan Englert
- PhUSE:
- Mission: Sharing ideas, tools and standards around data, statistical and reporting technologies to advance the future of life sciences.
- Liaison: Armin Schueler
- TransCelerate:
- Mission: TransCelerate BioPharma’s mission is to collaborate across the global biopharmaceutical research and development community to identify, prioritize, design, and facilitate the implementation of solutions designed to drive the efficient, effective and high-quality delivery of new medicines.
- Liaison: Pepa Polavieja and Helle Lynggaard
Contact
The general spirit of the working group is inclusive. If you’d like to contribute in one or the other way, we propose you first reach out to your company’s representative(s) (if applicable) and align within your company who is best placed to contribute.
For further information, please reach out to:
Chrissie Fletcher
chrissie.a.fletcher@gsk.com